THE PATENTS ACT, 1970
(39 of 1970)
&
The Patents [Amendments] Rules, 2006
GRANTED PATENT 283047
COMPLETE SPECIFICATION
(See section 10 and rule 13)
BIO-DIESEL FROM FATTY ACIDS OF MUSTARD OIL
Puri Oil Mills Ltd.
302, Jyoti Shikhar Building, District Center, Janak Puri,
New Delhi 110058
The following specification particularly describes and ascertains the nature of this invention and the
manner in which is to be performed.
1
Bio-Diesel from Fatty Acids of Mustard Oil
1. Technical Field of the Invention:
The present invention relates to novel process for developing biodiesel from fatty acids
derived from residual oil extracted from mustard meal. More particularly the invention
relates to the utilization of fatty acids from fat splitting and fractionation plant source after
separating the erucic acids and utilizing the remaining lower fatty-acids for conversion to
bio-diesel through trans - esterification.
The invention also provides for a system of plant lay-out for carrying out the above unit
operations of the developed process.
2. Background of the Invention and Prior Art
Biodiesel produced from vegetable oils as an alternative fuel has gained popularity in recent
years for its environment-friendly characteristics of low emission rates of CO, HC and CO2. A
variety of plant sources, such as soybean, rapeseed, canola, neem, jatropha as well as
animal fat are being widely explored in many parts of the world for their oil content as
feedstock for biodiesel. Rudolf Diesel, the inventor of first compression ignition engine for
which he was granted a patent in 1893, was himself a strong proponent of vegetable oil as a
potential source of fuel in the diesel engine. However, due to cheap and abundant
2
availability of petroleum based diesel, the use of vegetable oil as a fuel in the diesel engine
was pushed aside for more than hundred years.
The first public demonstration of vegetable oil based diesel fuel was sponsored by the
French government at a World Fair in 1900 using peanut oil. However, as the petroleum
based diesel engines evolved with newer and more efficient injection mechanisms, these
engine designs could not run on traditional vegetable oils primarily due to the much higher
viscosity of vegetable oils compared to petroleum diesel fuel. It was evident that a way was
needed to lower the viscosity of vegetable oils to make them suitable for use in modern
diesel engines.
It was in
1937 that a Belgian inventor proposed using trans-esterification to convert
vegetable oils into fatty acid methy esters
(FAME) and use them as a diesel fuel
replacement. Thus, the trans-esterification reaction is the basis for the production of
modern biodiesel.
The production of biodiesel is generally carried out by base catalysis of vegetable oils. The
first step of base catalyzed production of biodiesel process is mixing of alcohol and catalyst
using a standard agitator or mixer. The catalyst is typically sodium hydroxide (caustic soda)
or potassium hydroxide (potash). The alcohol/catalyst mix is then charged into a closed
reaction vessel and the oil or fat is added. The system from here on is totally closed to the
atmosphere to prevent the loss of alcohol. The reaction mixture is kept just above the
3
boiling point of the alcohol (around 70 °C) to speed up the reaction. Recommended reaction
time varies from 1 to 8 hours, and some systems recommend the reaction take place at
room temperature. Excess alcohol is normally used to ensure total conversion of the fat or
oil to its esters.
Precaution is required to monitor the amount of water and free fatty acids in the incoming
oil or fat. If the free fatty acid level or water level is too high it may cause problems with
soap formation and the separation of the glycerin byproduct downstream. Once the
reaction is complete, two major products exist: glycerin and biodiesel. Each has a
substantial amount of the excess methanol that was used in the reaction. The reacted
mixture is sometimes neutralized at this step. The glycerin phase is separated from
biodiesel phase by gravity and natural sedimentation or sometimes by centrifugation. The
excess alcohol is then removed with a flash evaporation process or by distillation.
Alternatively, the alcohol is removed and the mixture neutralized before the separation.
The recovered alcohol is re-used in the process.
The trans-esterification process as described above in nut-shell is the main process used for
biodiesel production since mid 19th century, though there are quite a few other processes
such as pyrolysis, micro-emulsion technique, direct blending etc. also known. In this trans-
esterification process, the triglyceride ester is reacted with alcohol (usually methanol) to
produce another ester (methyl ester) and alcohol (glycerol).
4
There are several patents in the prior art relating to the production of biodiesel from fatty
acids of vegetable oils. i.e., US patents 5482633, 5514820, 5536856, 5945529 and 6015440.
The processes described therein differ from each other in terms of source of vegetable oil,
choice of catalyst, reaction parameters, range of bye-products etc. The industries are taking
interest in commercializing some of these technologies on account of the techno-economic
viability of the production process and net operating returns of a representative plant which
co-produces a mix of bye-products such as glycerin, fatty acids, and filter-cake improving
the profit margins.
The present invention discloses a trans-esterification process of some of the fatty-acids
derived from mustard oil to produce fatty acids methyl ester (FAME) with very high
conversion rate for biodiesel and co-production of valued added erucic acids along with
conventional glycerol. The process of producing the end-product, biodiesel with a bye
product, erucic acid is ‘novel’ and ‘inventive’.
In India, Ministry of Non-Renewable Energy (MNRE), has been promoting the use of non-
edible oils for biodiesel production. Among the edible oils, mustard is important oil in India
which is widely used in most Indian house-holds and restaurants for cooking.
India produces more about 65 to 70 lakh tons of mustard annually which constitutes about
11% of the global production; nearly all of the oil seed with ~40% of oil content is processed
of which about 80% production is accounted for in the small scale sector. The overall yield
5
of the refined oil is generally around 30%; thus, approximately 20 lakh tons of refined oil is
available for domestic edible consumption. The remaining 10% of oil remains adhered to
the mustard cake which is extracted by alternate means but is usually unfit for human
consumption.
This invention is targeted to make good use of this resource which on country basis is
around 7 lakh tons currently and can easily be diverted to biodiesel production. The use of
this resource is likely to strengthen the country’s rural economy also with wide-scale
adoption of this technology like palm-oil is a major source of bio-diesel in Malaysia.
3. Object of Invention
The primary objective of the this invention is to develop a commercially viable process
technology including catalytic conversion method and plant lay-out equipment for the
production of bio-diesel (i.e., fatty acids methyl / ethyl ester) from erucic acid free fatty
acids derived from Indian mustard oil, more specifically from waste mustard oil.
Yet another objective of the present invention is to provide co-production of pure erucic
acid as a value added bye product of biodiesel production from mustard fatty acids.
4. Summary of Invention
6
The invention provides a method and system to obtain the biodiesel conforming to ASTM
and BIS standards with more than 98% conversion from fatty acids derived from Indian
mustard oil.
The main starting material of this invention is waste mustard oil or residual oil obtained
from mustard cake through solvent extraction. This mustard oil is split into glycerin and
fatty acids. The fatty acids are then fractionated into two major fractions as erucic acids
(C22: 1) and mixed fatty acids. The mixed fatty acids fraction which is essentially free from
erucic acids (C22:1) is converted to methyl ester through trans-esterification resulting into
Biodiesel of ASTM/BIS standard which can be used as a fuel for automotive engines direct or
as a blend with petroleum based diesel. The process disclosed herein provides Biodiesl of
very high quality along with two value added bye products, viz., erucic acid and glycerin. All
the resultant products of this invention after due purification are industrially useful and
together make the invention commercially viable.
5. Brief Description of the Accompanying Drawings
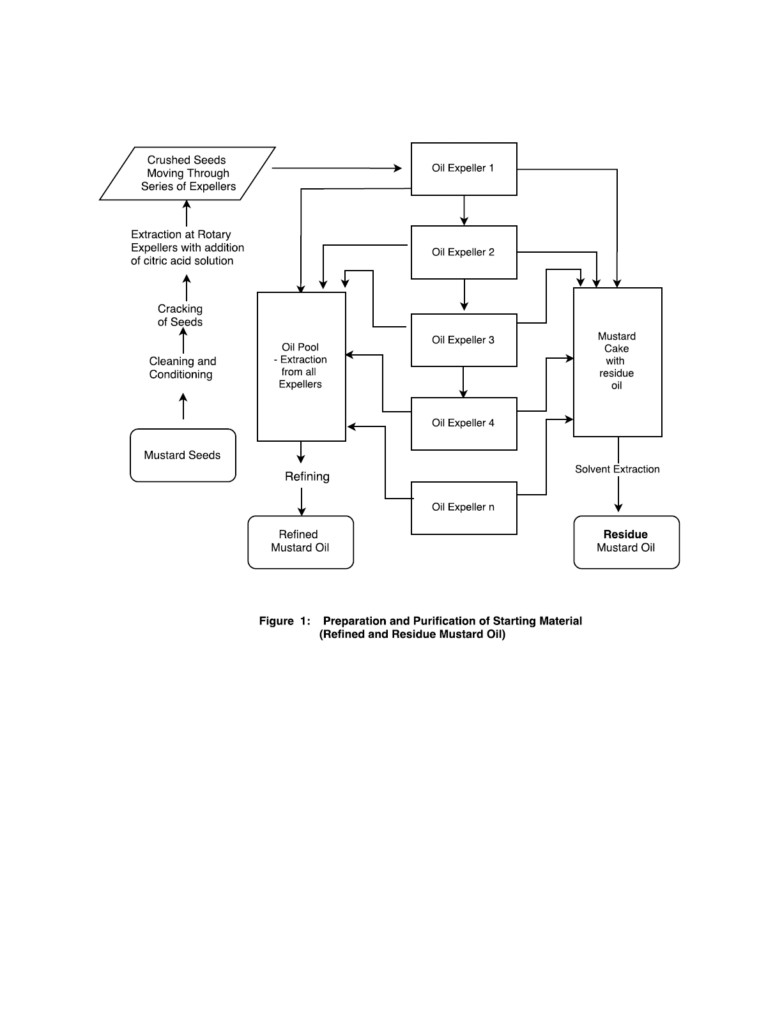
Figure 1 describes the steps of the process for preparing the starting material of this
invention viz., rresidue mustard oil.
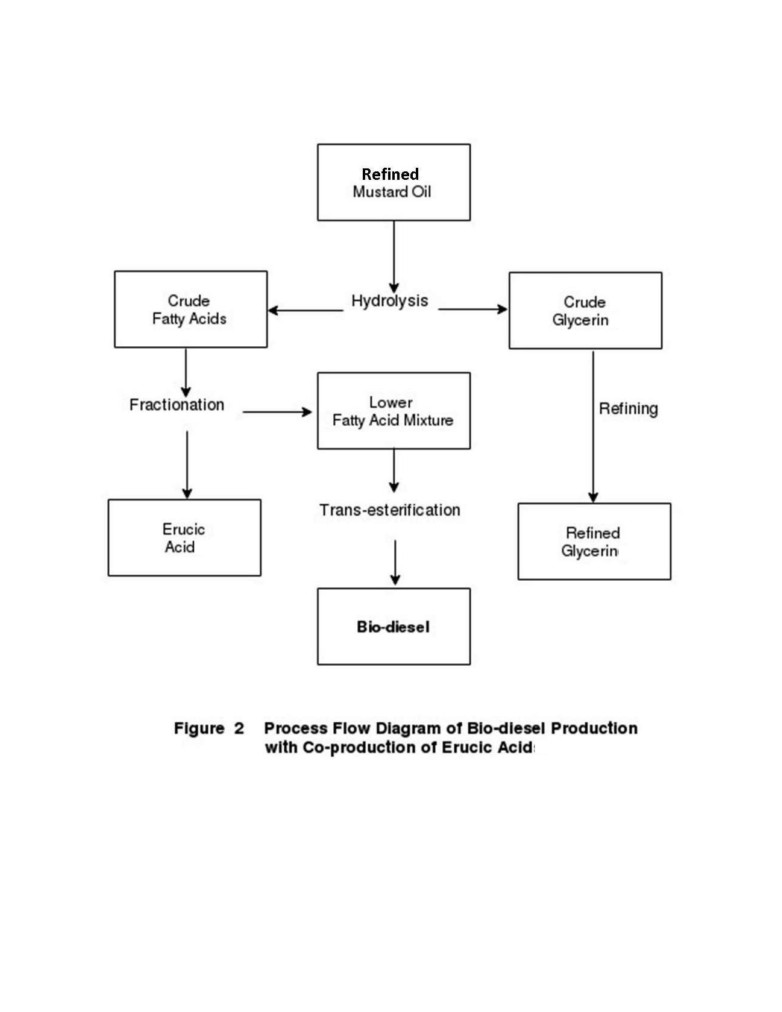
Figure 2 shows a process flow diagram for preparing biodiesel along with two bye products,
viz., erucic acids and glycerol starting from the residue mustard oil.
7
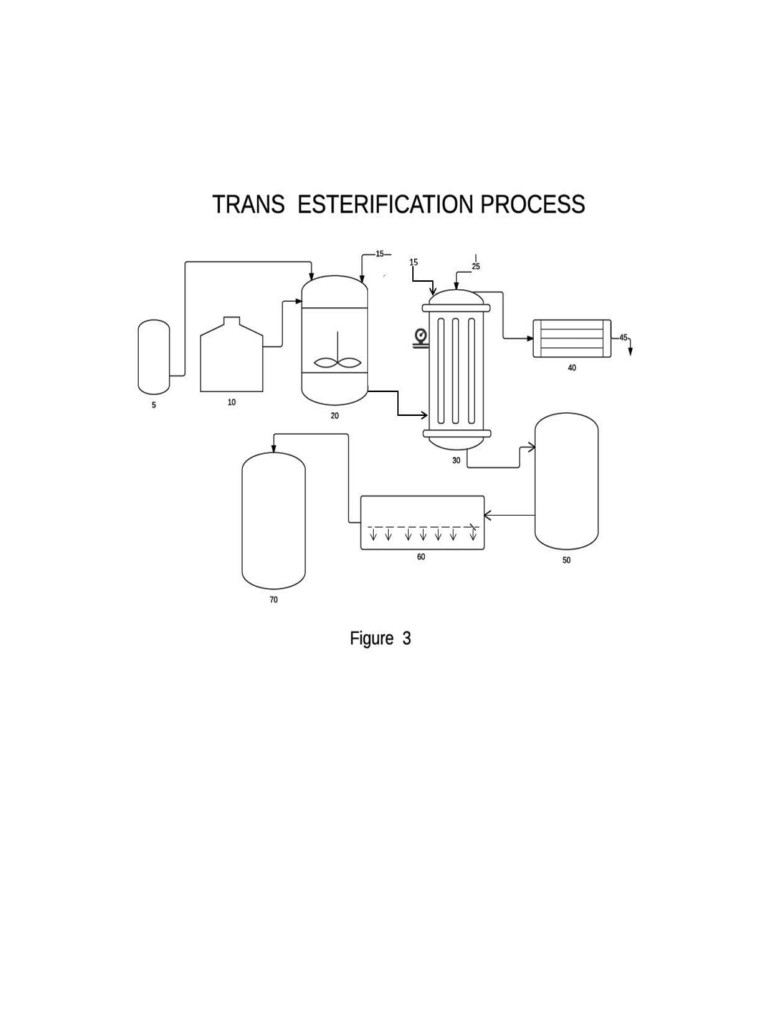
Figure 3 shows the process flow diagram of the trans-esterification process of this invention
in one embodiment
6. Detailed Description of the Invention
Problem Identification & Technological Solution
In view of widespread use of variety of vegetable oil for biodiesel production, it was
obvious to explore the use of mustard oil for this purpose which has not been tried hitherto
and is plentiful in India. The developer of this technology and applicant of this invention is
research driven company and is one of the major producers and supplier of mustard oil
throughout India.
As already stated before, most vegetable oils exist in high viscosity range so that these
cannot be used directly in conventional engines. Thus, these are necessarily converted to
methyl esters by trans-esterification process resulting into a product of lower viscosity
suitable for most internal combustion engines with no or minor modification. Unlike most
other vegetable oils, however, mustard oil contains more long-chain fatty acids and
therefore biodiesel derived from it turns out to be slightly more viscous (thicker) and has
major technological challenge in the use of mustard oil for biodiesel production.
8
In order to find a workable solution to the problem, the composition of various fatty acids
in mustard oil was looked at in comparison to other vegetable oils. It was found, that
mustard oil contains ~22-50 % Erucic acid, a (C22:1) fraction and ~5-13 % of Ecosenic acid, a
(C20:1) fraction, which are normally not present in other commonly available vegetable oils
used in biodiesel production. The remaining fatty acids, namely linolenic acid (C18:3) (6-
18%), linoleic acid (18:2) (10-24%), oleic acid (C18:1) (8 - 23%), stearic acid (C18:0) (0.5-2%)
and palmitic acid (C16:0) (0.5-4.5 %) are present in mustard oil as in most other vegetable
oils, such as sunflower, soybean, palm etc. albeit in varying quantities.
Novelty & Inventiveness
In view of the above, separation of some of these longer chain fatty acids, particularly
erucic acid through fractionation before trans-esterification of the remainder of fatty-acids
mixture was considered as a possible solution. This approach proved fortuitous and
worked well. The process as disclosed is both ‘novel’ as well as ‘inventive’ as i) biodiesel
from mustard oil and ii) erucic acid co-production with biodiesel are not hitherto reported
in scientific and patent literature.
While many facets of the technology disclosed herein are in the realm of prior art, such as
oil refinement, fat splitting and fractionation, the process of trans-esterification as
reported herein that provides enhanced surface-area and possible innovations with regard
9
to implementing the technology by way of process engineering, related to choice of
catalyst, equipment and optimization of reaction conditions are quite unique. All inventive
features of the developed technology will be evident from the description of complete
specification that follows here with the help of accompanying drawings.
Other objects and aspects of the invention will become apparent from the following
description and the embodiments with reference to the accompanying drawings, which is
set forth hereinafter. The embodiments of the present invention can be modified variously.
Thus, the scope of the present invention should be construed not limited to the description
and embodiments provided herein. The embodiments are provided to better explain the
present invention to those of ordinary skill in the art.
Reference Numerals in the drawings (Figure 3)
5 = Alcohol (Methyl / Ethyl alcohol) Tank
10 = Free Fatty Acid Tank
15 = Catalyst inlet
20 = Vessel for mixing and emulsification
25 = Steam inlet
30 = Pressure Reactor with steam inlet
40 = Steam / water condenser
45 = Outlet for condensed water
50 = Settling tank
10
60 = Cross flow or multi-phase filter
70 = Collection vessel for crude fatty acid methyl ester (Biodiesel)
Process Description
The process starts from residue mustard oil which is obtained as shown in the Figure 1 .
The further process steps starting from this oil are shown in a flow diagram in the Figure 2.
Fat Splitting: The very first step in Figure 2 is ‘fat-splitting’ operation which essentially is a
hydrolysis process. Mustard oil is pumped from the storage tank to the splitting plant via a
steam based pre-heater. The heated oil enters the pressurized splitting column from the
bottom of the splitting column. Demineralized water is fed to the top of the column with a
high pressure pump resulting in a countercurrent flow. High pressure steam is injected into
the column to provide the necessary heat. A pressure of about 55 bar is maintained along
with a temperature of above 250°C. The process is continuous and glycerin water is
continuously discharged from the bottom while split fatty acids are discharged from the
top of the splitting column. The fatty acid mixture is cooled and dried in a flash evaporator.
Similarly, glycerin water goes to a flash-evaporator and the condensate collected and
reused as process water.
Fatty Acid Fractionation
11
The erucic acid component in the fatty acids mixture is separated from the remaining fatty
acids in a custom built fractionating column. The fractional distillation returns the erucic
acid of 90% or more of purity.
Trans-Esterification of Fatty Acid Mixture
The main process steps of trans-esterification along with equipment lay-out are depicted in
Figure 3. In the disclosed process the free fatty acids is remaining after separating the
erucic acid component are converted by acid esterification into fatty acid methyl or ethyl
esters in presence of catalyst sulphuric acid at 180 - 280 °C under pressure of 400 - 1 OOO
kPa. The conversion rate of this process is 99%. The equipments used are acid resistant
containers such as stainless steel containers.
Example
The conversion of fatty acids to biodiesel was carried out in an experimental set-up as
shown in Figure 3 using the following process:
Free fatty acids (10) from fat splitting plant were taken and analyzed for their acid value,
triglyceride content etc. The fatty acids from (10) were then mixed with alcohol (5) such as
methanol or ethanol from 5-35% on free fatty acid weight basis and a proper stirring or
12
blending was carried out in (20) at 100 - 300 rpm, preferably at 200 - 250 rpm, for proper
mixing of the alcohol (methanol) and fatty acids.
Acid catalyst such as sulphuric acid (15) is added at the rate of 0.1-2% and further stirring is
done. The whole mixture is then loaded in a pressure reactor (30), which is heated with live
steam (25), the pressure of the chamber being 400 - 1OOO kPa, preferably 800 - 1000 kPa.
The reaction was continued for a minimum of 30 min to up to 3 hours. The output of this
process is fatty acid ester (methyl or ethyl), sulphuric acid, water and traces of some
triglyceride.
In this process, the water (45) is removed as steam condenses in a condenser (40) attached
to the main pressure reactor (30). The water (45) is continuously removed. The water
vapour out-flow has been observed to take away a mist of acid catalyst with it. Thus,
additional quantities of acid catalyst at the rate of 0.1 - 1% is required to be fed during the
reaction. The methyl or ethyl ester of fatty acids mixture obtained on completion of the
reaction is transferred to a settling tank (50).
To work out the reaction product of methyl ester of the fatty acids settled in (50), water
wash was employed to remove acidic impurities followed by filtration through cross-flow
filtration (60). This important process step allows a separation of the individual phases of
13
the mixture followed by water washing and drying of the final product of biodiesel which
conforms to ASTM and BIS standards.
Possible Embodiments of Equipment and Plant Lay-out
There are many possible embodiments of equipment layout to perform this invention and
obtain desired results, some of which are described below.
1. In this embodiment of the invention, the separation of the emulsion phases is
performed by exploiting the surface forces in a well-designed cross-flow filter. In
another embodiment of the invention, the multiple phases of the emulsion were
separated by multiphase filtration. Thereby, the water was separated in the first step,
and the triglycerides were separated as residue in the second step. In a third step,
methanol is separated from the fatty acid methyl ester.
2. In yet another embodiment of the invention, distillation can be carried out directly on
the reaction product of (50) after chemical balance state is reached, possibly after
separation of the phases of the mixture employing vacuum distillation at a much
reduced temperature.
3. In another embodiment, multiphase distillation on the the reaction product from
pressurized reactor (30) employing evaporation, in particular down-flow evaporation.
14
4. The aim of the invention is realized independently by judicious choice of equipment for
implementation of the process. The equipment as required to perform the invention
includes the following -
a. at least one container for the fatty acids, and
b. at least one source tank each for the catalyst sulphuric acid solution, and the
alcohol,
c. at least one mixing vessel for compounding,
d. at least one pressurized reaction vessel connected with a boiler for live steam.
e. a unit for separating the phases of the emulsion downstream from the reaction
section.
f. a high pressure pump for injecting the emulsified feed with a turbulence into
the pressurized reactor
5. In yet another embodiment of equipment required for the invention, following may
include -
a. a dynamic separator for creating and managing liquid-liquid emulsions.
b. a cross-flow or multi-phase filtration unit or alternatively a surface filter
designed as a plate filter with cotton cloth appropriate pore size.
c. a distillation unit comprising of an evaporator and a condenser in the
downstream section of the main reactor, alternatively,
d. a down-flow evaporator or a vacuum evaporator or a thin-layer evaporator.
15
While the present invention has been described with respect to certain preferred
embodiments, it will be apparent to those skilled in the art that various changes and
modifications may be made without departing from the scope of the invention as
defined in the above examples of the possible embodiments.
Mode of Enablement
To enable this invention to perform, besides preparing the product and optimizing the
production technology, it is also important to characterize and test the quality of the
product for which purpose it is made. The biodiesel as prepared from a fatty acid fraction
devoid of erucic acid was therefore subjected to various tests as prescribed by BIS and
ASTM for biodiesel and the same were compared with biodiesel sample prepared from
mustard oil fatty acids (without separating erucic acid from it). The results as obtained are
tabulated below in Table 1. It was found, as expected, that the product obtained by fatty
acid fraction having no erucic acid in it gave a much superior product with lower viscosity
and better fuel characteristics than that was obtained from a fatty acid mixture obtained
from mustard oil from which the erucic acid was not separated.
A preliminary assessment shows that the biodiesel of this invention is way better than the
normal biodiesel prepared from mustard oil and can as well be used as automotive fuel
without much blending with petroleum diesel.
Dr Rajendra Prasad
IN/PA-1498
Agent for Applicant
16
7. Claims
We claim:
1. A process for obtaining biodiesel from fatty acids mixture (obtained from Indian
mustard oil from which erucic acid, the C 22:1 component has been previously
removed) comprising of:
a) mixing of fatty acids with methanol or ethanol in a ratio of
95:5 to 65:35,
b) adding to the mixture, sulphuric acid catalyst in amount of
0.1 - 5% on fatty acid weight basis,
c) mixing the reactants at 100-300 rpm to create finest possible
emulsion and optimum dispersion of fatty acids in the
alcoholic medium,
d) raising the temperature to 180-280°C and raising the pressure from to 800
kPa - 1000 kPa, and
e) stirring of the mixture for about 30 minutes to 3 hours.
2. A process for obtaining biodiesel from fatty acids obtained from Indian mustard oil
in which erucic acid, the c22:1 component is co-produced by distillation through
fractionating column from the fatty acid mixture of the mustard oil prior to
subjecting the mixture to trans-esterification.
17
3. A process for obtaining biodiesel from fatty acids of mustard oil as claimed in claim
1 wherein the said acid catalyst is sulphuric acid.
4. A process for obtaining biodiesel from fatty acids of mustard oil as claimed in claim
1 wherein
i)
the trans-esterification reaction is carried out in a pressurized reactor by
passing hot live steam and its condensate water is continuously removed from
the reactor,
ii)
the pressure is maintained between 800 kPa - 1000 kPa and
iii)
acid catalyst (sulphuric acid) is periodically charged into the reactor at the rate
of 0.1 - 1% to make up for the lost acid with the condensed steam
5. A process for obtaining biodiesel from fatty acids of mustard oil as claimed in claim
1 wherein the biodiesel obtained at the end of the reaction which separates out in
upper layer is purified by filtration to remove suspended solid particles.
6. A process for obtaining biodiesel from fatty acids of mustard oil as claimed in claim
5 wherein said the filtrate containing biodiesel is subjected to evaporation to
remove excess alcohol which is reused in the reaction.
7. A process for obtaining biodiesel from fatty acids of mustard oil as claimed in claim
5 or 6 wherein the filtered biodiesel is subjected to washing with water to remove
traces of acid catalyst.
18
8. A process for obtaining biodiesel from fatty acids of mustard oil as claimed in claim
7 wherein said biodiesel is subjected to caustic stripping to remove any unreacted
fatty acids or triglycerides.
----------
Dr Rajendra Prasad
IN/PA-1498
Agent for Applicant
19
ABSTRACT
BIO-DIESEL FROM FATTY ACIDS OF MUSTARD OIL
A process and system of equipment and plant lay out for obtaining bio-diesel from
fatty acids from Indian mustard oil with the help of acid catalyst is disclosed. The
process co-produces erucic acid, a C22:1 component of the fatty acids mixture in a very
pure form as an additional bye-product besides glycerol. The biodiesel obtained from
the process is very clean and pure and conforms to the BIS standards. The bye product,
erucic acid together with glycerol renders the Bio-diesel production from this process
highly techno-economically viable.
Dr Rajendra Prasad
Dated 28th day of October 2015
IN/PA-1498
Agent for Applicant
20
Puri Oil Mills
Sheet 1 of 4
Dr Rajendra Prasad
IN/PA-1498
Agent for Applicant
21
Puri Oil Mills
Sheet 2 of 4
Dr Rajendra Prasad
IN/PA-1498
Agent for Applicant
22
Puri Oil Mills
Sheet 3 of 4
Dr Rajendra Prasad
IN/PA-1498
Agent for Applicant
23
Puri Oil Mills
Sheet 4 of 4
Sheet 4 of 4
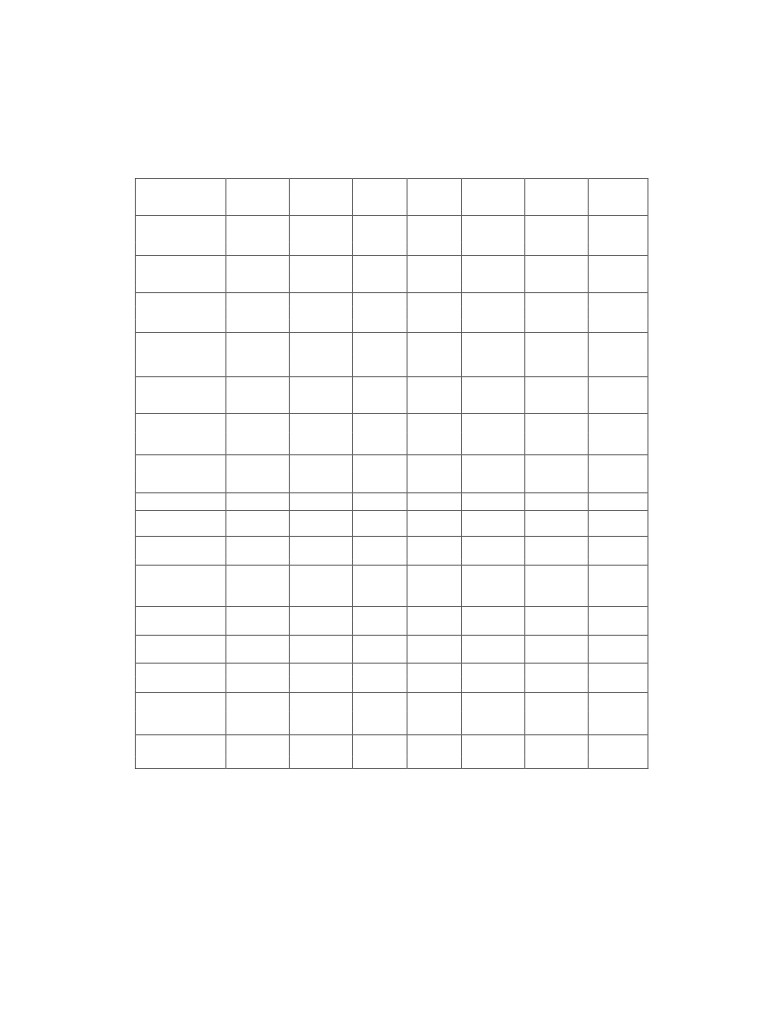
TEST RESULTS FOR BIODIESEL OF THIS INVENTION
TEST
BIS
BIS
ASTM
ASTM
Biodiesel
Biodiesel
Remarks
PROPERTY
Method
Limits
Method
Limits
(M)
(MFA - EF)
IS 1448,
Flash point °C)
Min 120
D-93
Min 130
167.3
175
OK
P:21
Phosphourus
D-4951
Max
D-4951
Max
< 10 ppm
< 10 ppm
OK
(ppm)
10 ppm
10 ppm
Water/Water &
D-2709
Max
D-2709
Max
Nil
Nil
OK
sediment (ppm)
ISO 3733
500 ppm
500
ISO 6296
ppm
CCR 100%
D-4530 /
Max
D-4530
Max
0.012
Nil
OK
(% mass)
ISO10370
0.050
0.050
Sulphated ash
ISO 6245
Max
D-874
Max
0.014
0.014
OK
(% mass)
0.02
0.020
Kin. Viscosity at
ISO 3104
2.5
- 6.0
D-445
1.9
- 6.0
5.80
4.35
OK
40 °C (cst)
Sulphur (ppm)
D-5453
Max
D-5453
Max
< 10 ppm
< 10 ppm
OK
50 ppm
500 ppm
Cetane number
ISO 5156
Min 51
D-613
Min 47
60.4
57
OK
Copper corrosion
ISO 2160
Max 1
D-130
Max 3
No.1
1
OK
Neutralization
IS 1448,
Max 0.50
D-664
Max 0.80
0.04
0.07
OK
Value
P:1/Sec1
Free glycerin
D-6584
Max
D-6584
Max
0.014
0.014
OK
(%mass)
0.020
00.20
Total glycerin
D-6584
Max
D-6584
Max
0.014
0.016
OK
(% mass)
0.250
0.240
Distillation
D1160
90% at
399 °C
358 °C
*
Temp. (°C)
360°C
Oxidation Stability
EN 41121
Min.6 hr.
-
-
5.84
0.52
**
Density at 15°C
ISO 3675
860 - 900
-
-
885
885
OK
kg/m3
ISO 12185
Methanol, % by
EN14110
0.20
-
-
0.04
0.022
OK
mass, max
*Fail ASTM - Biodiesel(M)
Biodiesel (M) = Biodiesel of Fatty acids of Mustard Oil
**Fail BIS
Biodiesel (MFA-EA) = Biodiesel of Fatty Acids of Mustard
Oil Devoid of Erucic Acid
Table I
Dr Rajendra Prasad
IN/PA-1498
Agent for Applicant